【法規工具文-小結】 Quick-guide on Software Pre-Cert Program (6) — Summary
【導讀】本文最初用英文寫作,目的是保留FDA原意。而本版的目的為工具用文章,故用中文註釋以便快速抓到重點,但細節部分還是建議細讀英文說明
因為這是用規格書的手法來撰寫,建議讀者從結論的Fig 3先了解已得出清楚的輪廓。而再從4個元件逐一了解。
這個系列的使用方法建議如下
(1)請先參考第一篇導論,了解整個Program的目的及基本核心架構
(2)參考本篇,了解這個Program的要求為何
(3)最後,在第2,3,4,5等篇的細部介紹,了解其細節。
In this article, the author summarized the Pre-Cert Program in the form of the requirements in the four components. It can be considered as the specifications of both organization and product level for SaMD company.

Introduction
The Pre-Cert program is executed in TPLC approach with four components shown as Fig 1. The requirements at each component are shown in the following sections.
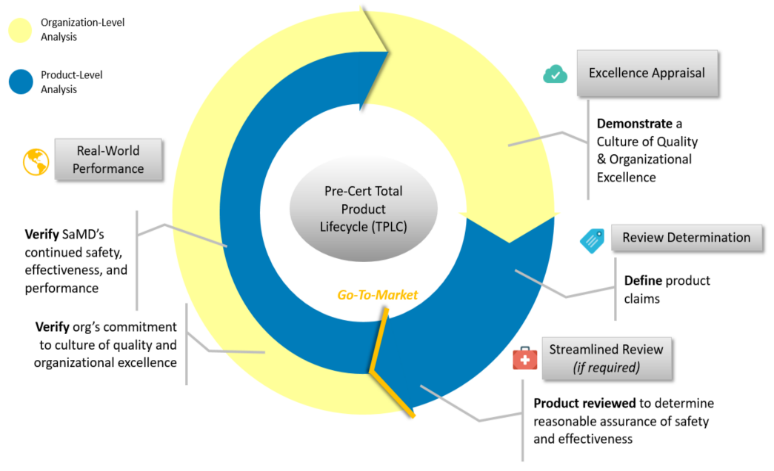
Excellence Appraisal
Requirements from FDA:
Domains & Elements:
- 1. Leadership and Organizational Support — Elements related to the organization’s leadership establishing the strategic direction, responsibility, authority, and communication to assure the safe and effective performance of the SaMD.
- 2. Transparency — Elements related to the organization’s open sharing of relevant information with all stakeholders to build confidence in the organization and its products.
- 3. People — Elements related to providing appropriate resources as needed for ensuring the effectiveness across all lifecycle processes and activities in meeting user requirements.
- 4. Infrastructure and Work Environment — Elements related to the availability of infrastructure such as equipment, information, communication networks, tools, and the physical facility throughout SaMD lifecycle processes.
- 5. Risk Management: A Patient Safety Focus — Elements related to monitoring and managing risks along multiple dimensions such as user-based, application-based, device-based, use environment-based, and security-based across all lifecycle processes.
- 6. Configuration Management and Change Control — Elements related to identifying and defining the software configuration and controlling the release and change of the software throughout all lifecycle processes.
- 7. Measurement, Analysis, and Continuous Improvement of Processes and Products– Elements related to managing and improving product realization and use through real- world performance monitoring.
- 8. Managing Outsourced Processes, Activities, and Products — Elements related to understanding, maintaining control, and managing the effect of outsourced activities, processes or products.
- 9. Requirements Management — Elements related to clear, and often repeated user interaction to understand and clearly articulate user needs throughout all lifecycle processes.
- 10. Design and Development — Elements related to ensuring safe, effective, and secure SaMD based on user and other performance requirements during all lifecycle phases at key milestones and good development practices incorporating appropriate review activities such as code review, peer review, and self-review.
- 11. Verification and Validation — Elements related to understanding the criticality and impact to patient safety by providing assurance of conformity to requirements and reasonable confidence that the software meets its intended use/user needs and operational requirements.
- 12. Deployment and Maintenance — Elements related to activities such as delivery, installation, setup, and configuration of software including documentation and user training materials that identify any limitation of the algorithm, provenance of data used, assumptions made, etc. that should be considered during deployment. Additionally, modification of previously deployed software while preserving the integrity of the software by not introducing new safety, effectiveness, performance, and security hazards.
Suggested Submission Material from Organization:
Example of activities/processes and KPIs related the element of the Excellence Appraisal by organization is shown as Table 1.
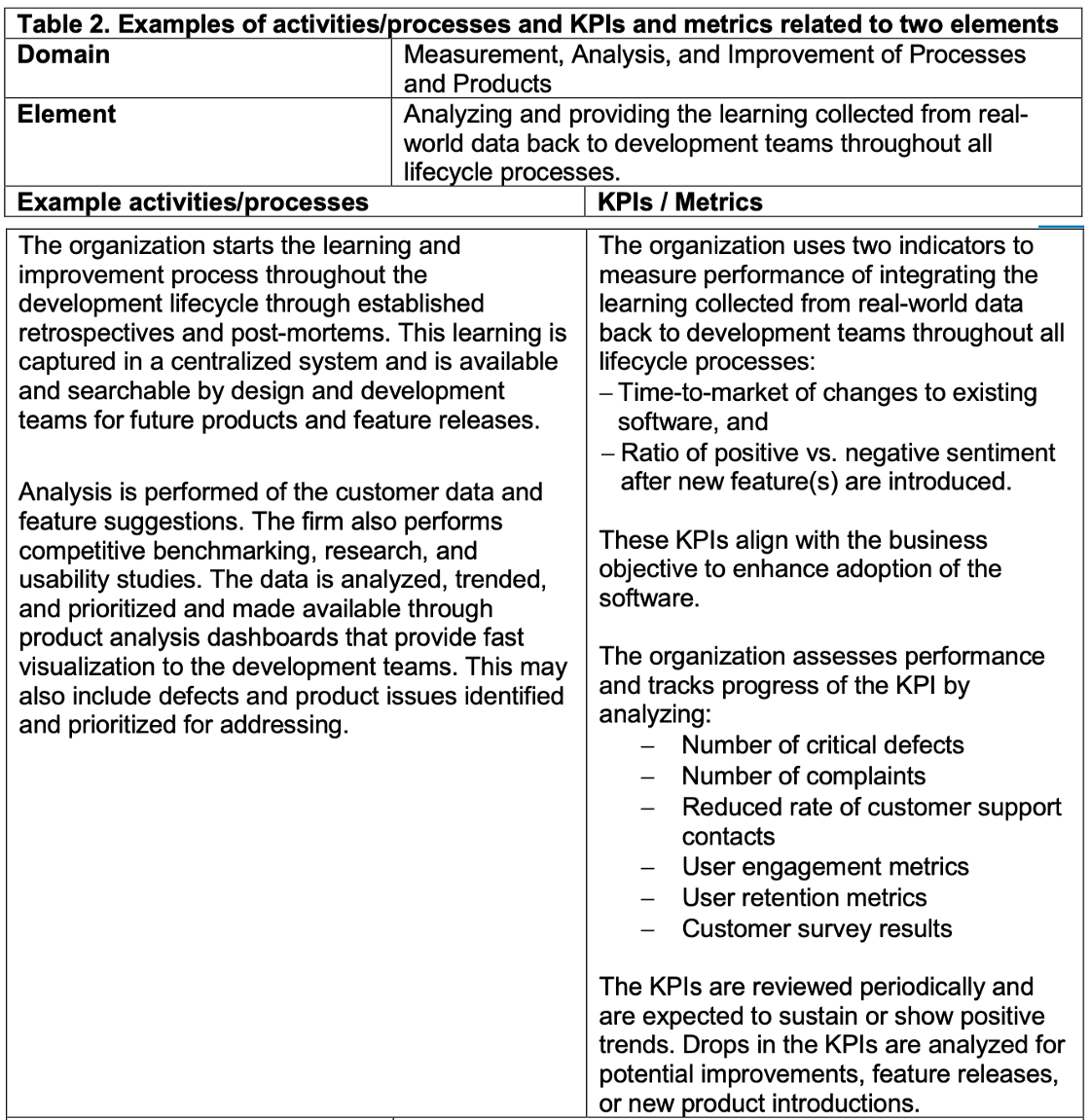
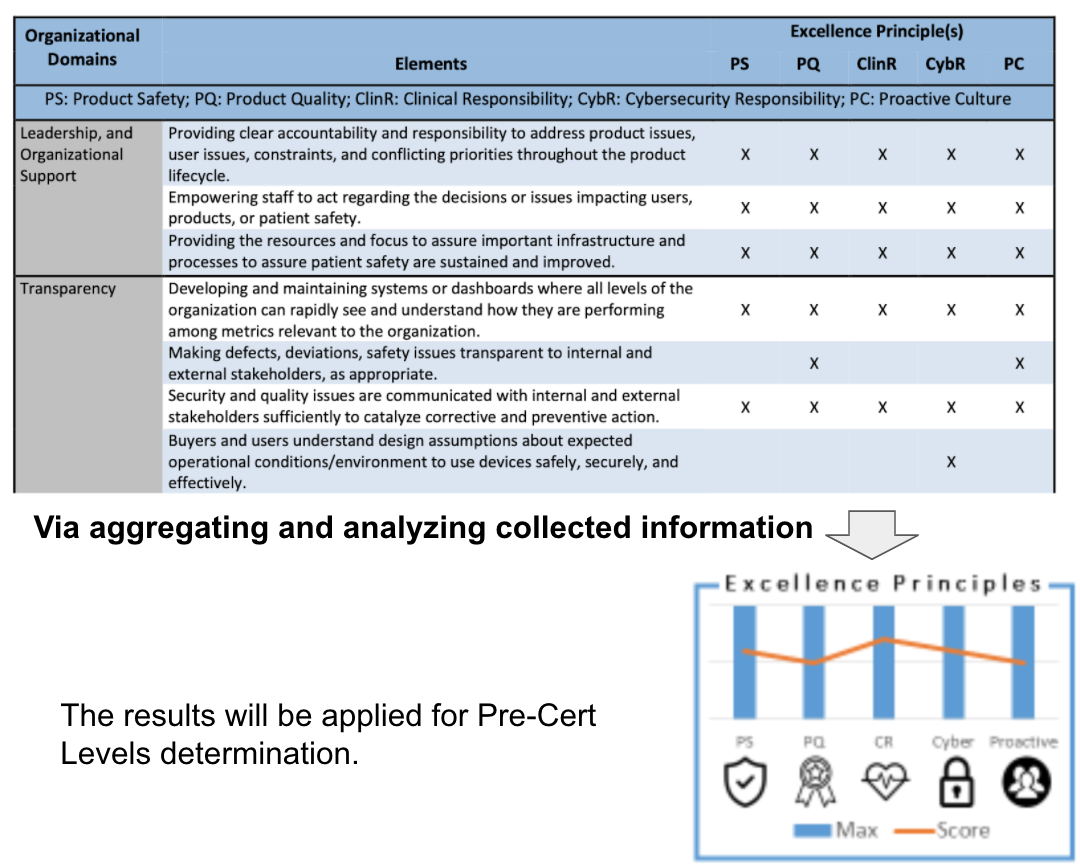
Assessment example by FDA, illustrated as Table 2, and results as Fig 2.
It contains domains , elements, and appraisal against CQOE. Then level 1 or level 2 will be determined by aggregating and analyzing collected information.
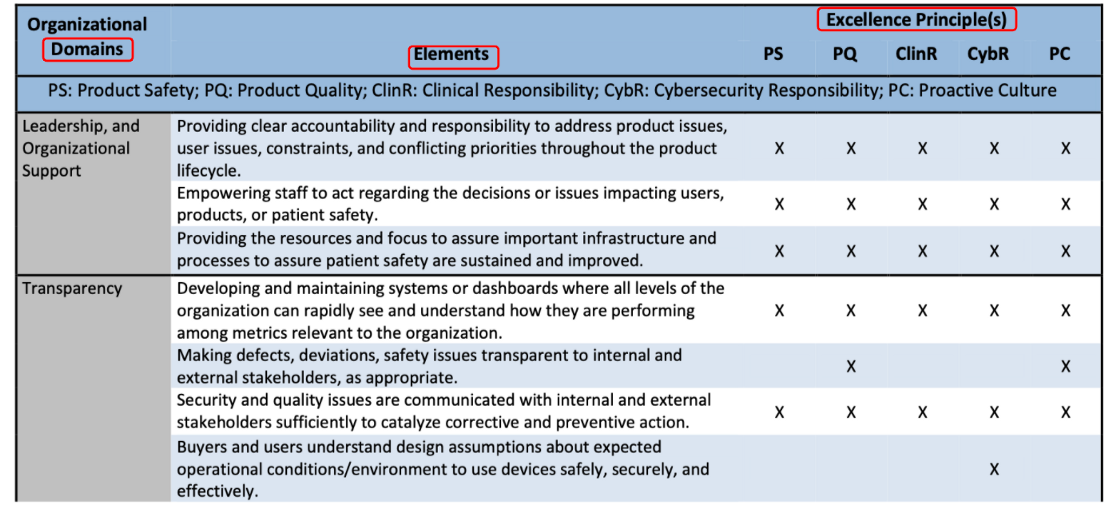
【導讀】最後Excellence Appraisal的評分手法可從Fig 2了解,其就12個Domain,以COQE的要求評分,最後加總得出總評。
Review Pathway Determination
Based on the excellence appraisal assessment results, Level 1 and Level 2 will lead to different path shown as Table 3.
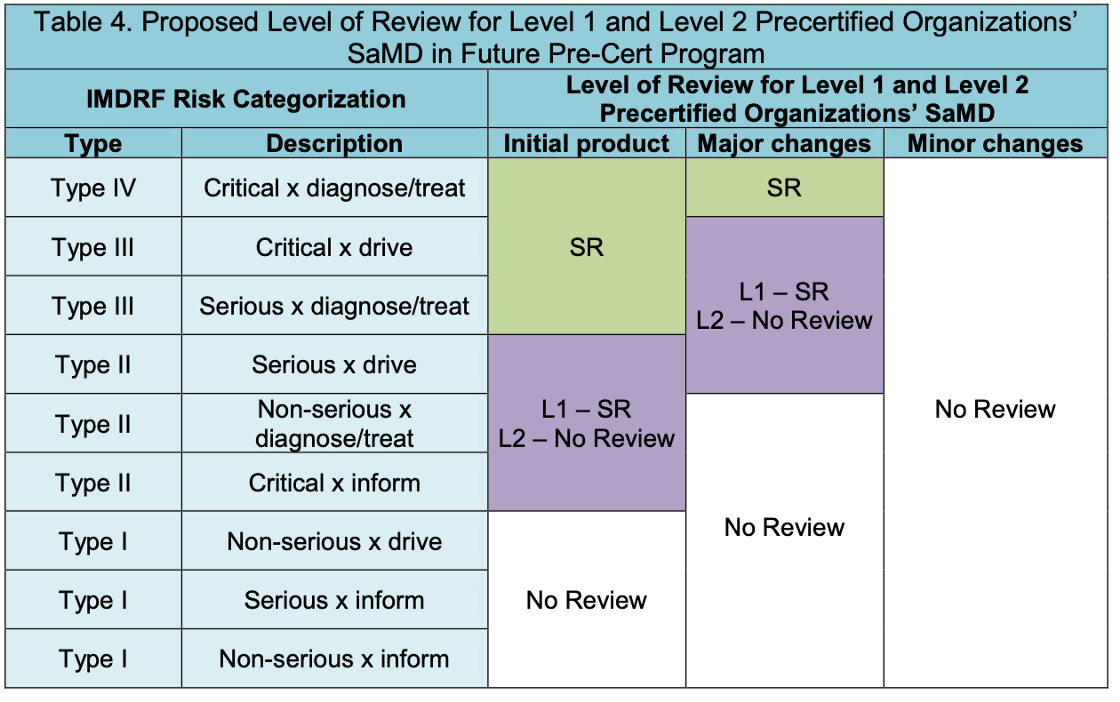
Streamlined Review
Review elements:
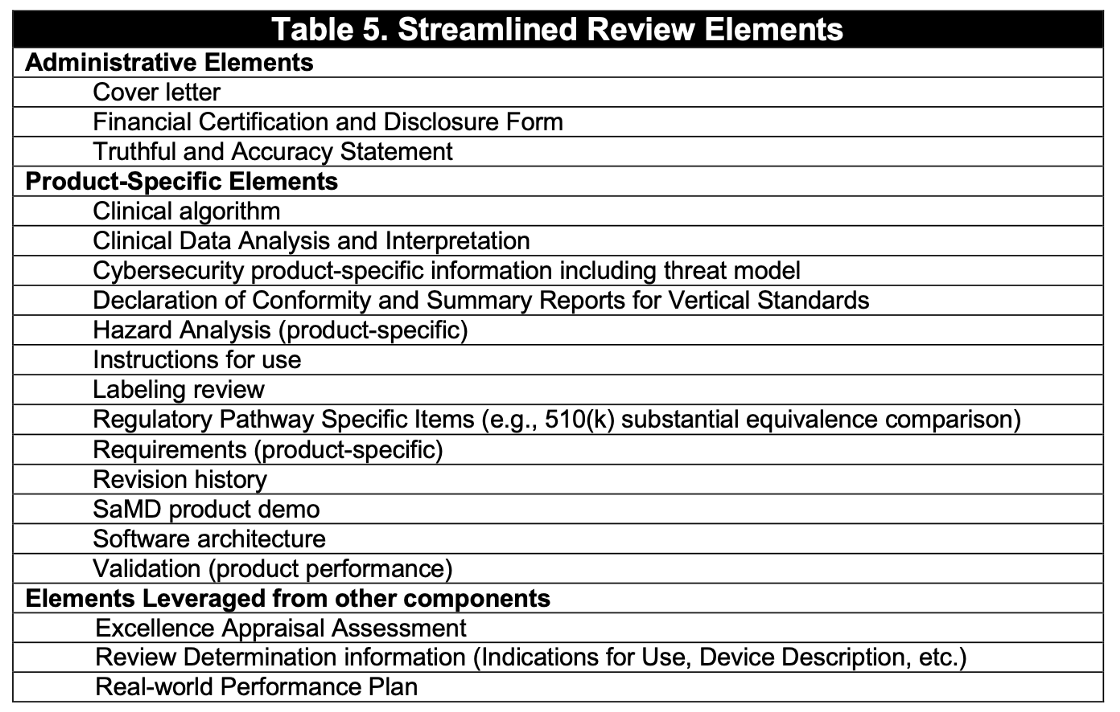
Real World Performance
The FDA considers RWPA to encompass at least three types of analyses, RWHA, UXA, and PPA.
The followings are the illustration about how these 3 domains and its subdomains are assessed against CQOE principles.
Real-World Health Analytics (RWHA) are defined as analyses of real-world clinical outputs and outcomes related to the intended use of the SaMD product.
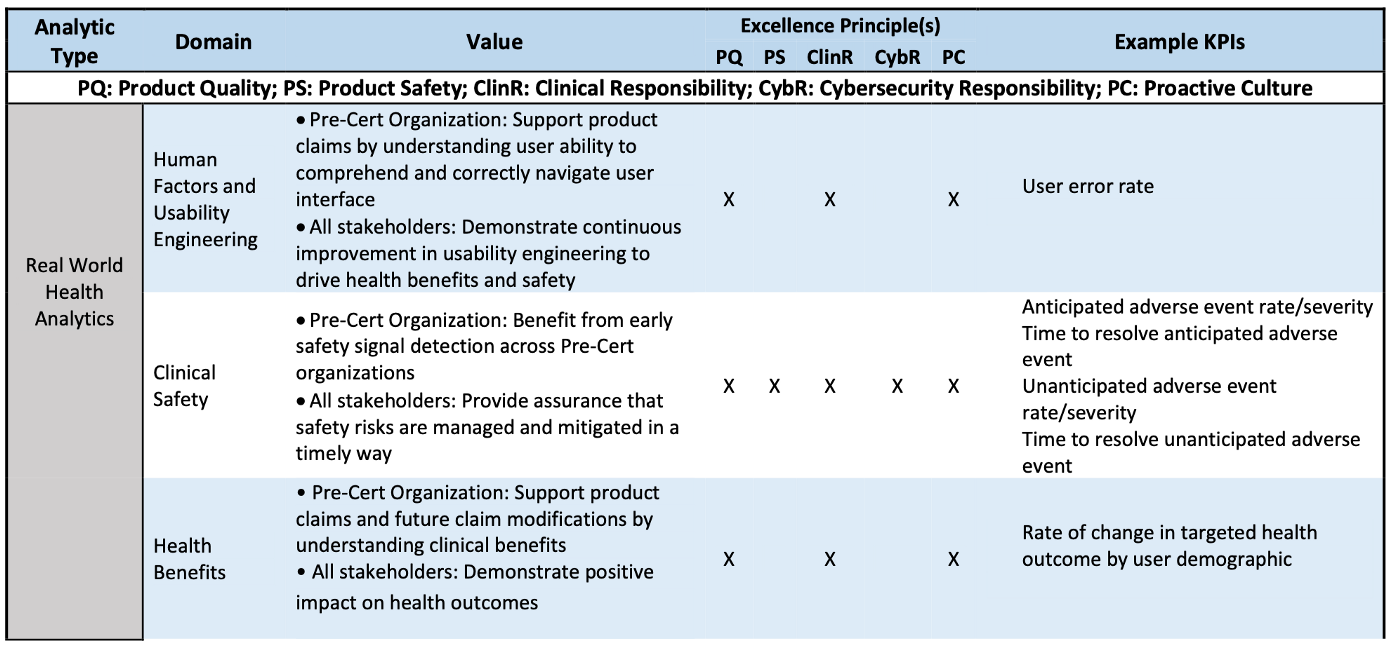
User Experience Analytics (UXA) are defined as analyses of user experience outputs related to the real-world use of a SaMD product.
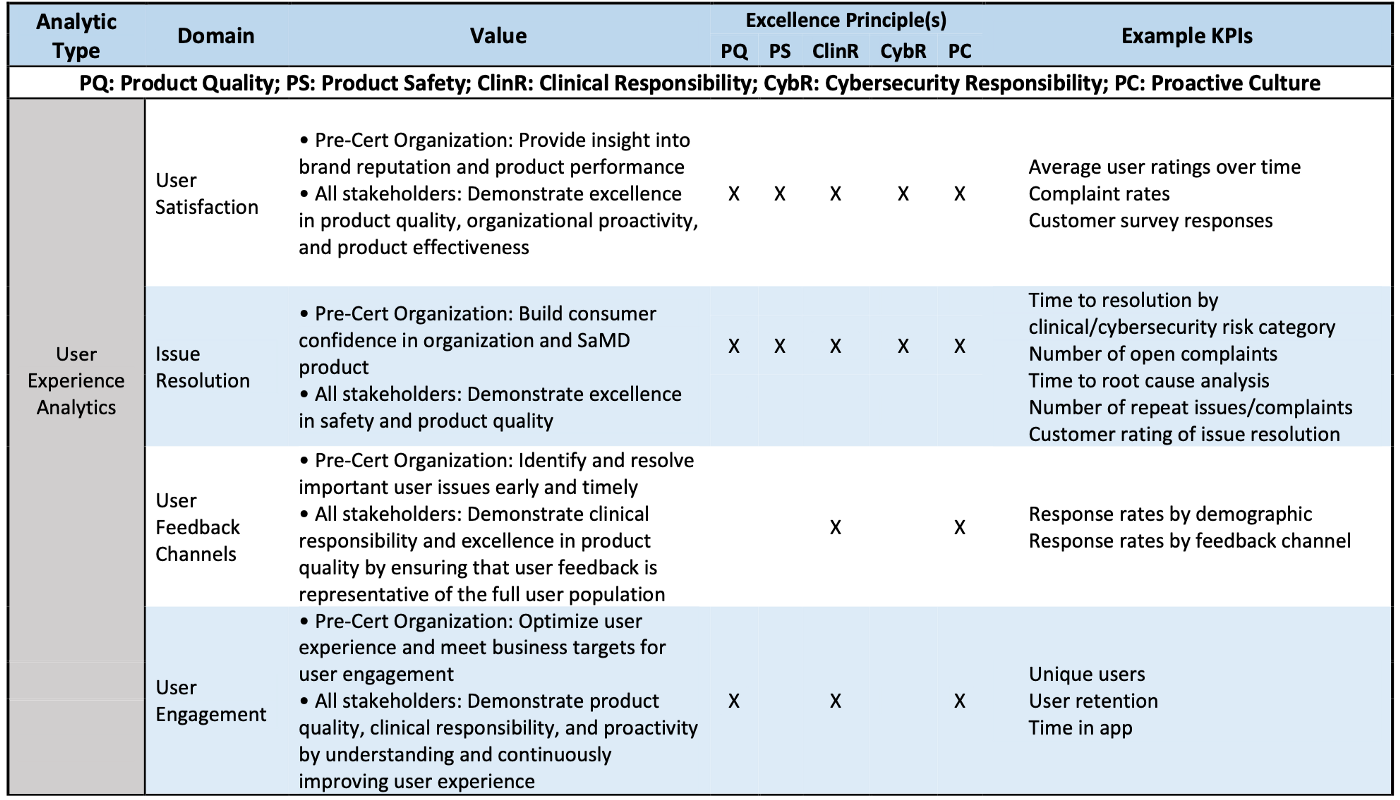
Product Performance Analytics (PPA) are defined as analyses of outputs and outcomes demonstrating the real-world accuracy, reliability, and security of a SaMD product.
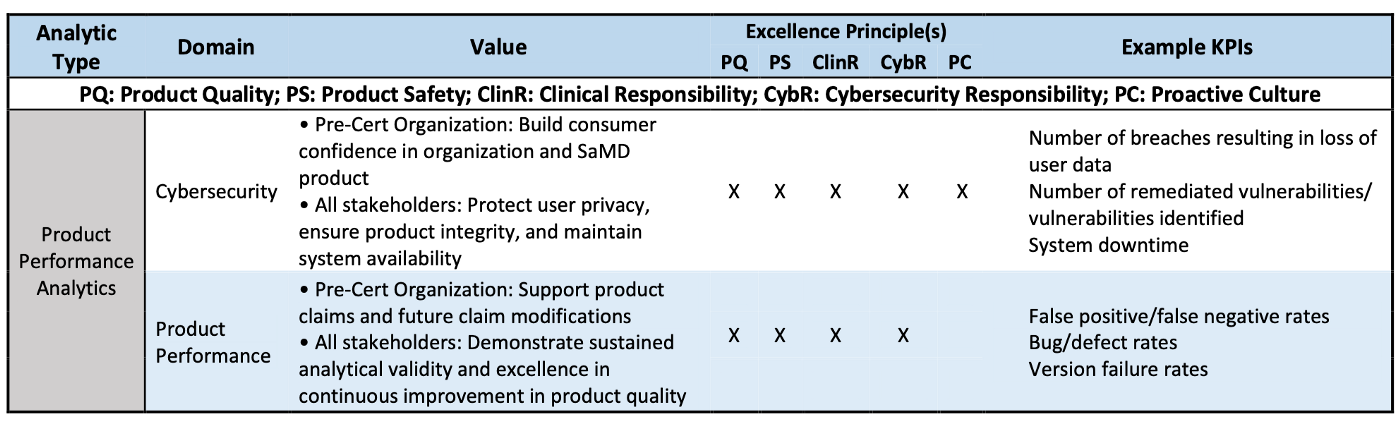
Summary
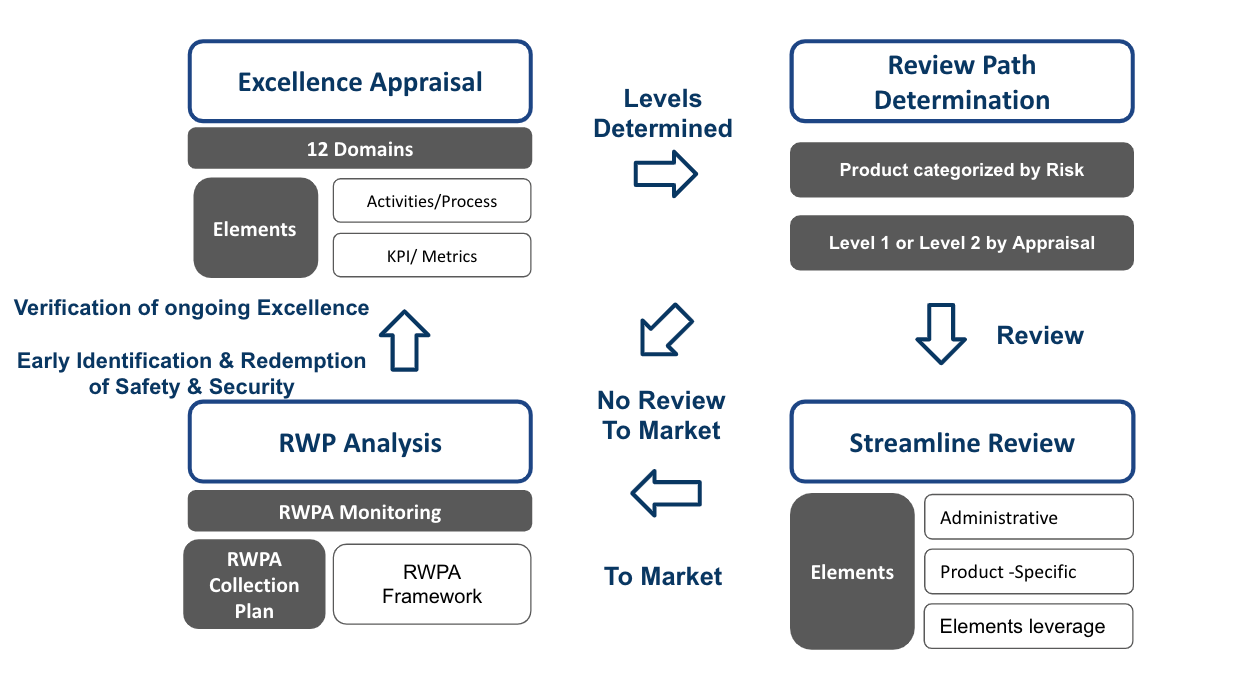
【導讀】以下Fig 3,為整個需求的總覽
The requirements in the each component can be summarized as Fig 3.
【導讀】以下討論Pre-Cert Program與現行機制的主要不同。其可發現,對組織卓越性要求的比重相當高,所以未來開發SaMD的公司,一定要在組織上下不小功夫。
In comparison to traditional review process, significant difference are found as following.
- Excellence Appraisal: Transparency , Configuration management, and Requirement management.
- Review pathway determination: Unlike slight change may be subjected for special 510K review, flexibility review for design changed applied.
- Streamlined Review: The best description for this improvement is similar to the “focused review” for AI/ML SaMD.
- Real World Performance Analysis: It is one of the significant change. This concept is aligned with Post-Market surveillance (PMS) and Post Market Clinical Follow-UP of MDR or IVDR.
Significant effort are found in organizational level. Next, author will make the comparison with the proposed framework for AI/ML SaMD. The final goal will be the discussion of coping strategy for the fabrication of the organization meeting the requirements.
